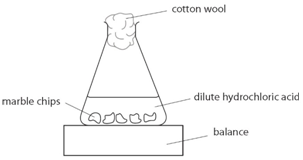
Investigating the rate of reaction between marble chips and the varying concentrations of hydrochloric acid 705 words 3 pages.
Marble chips and hydrochloric acid experiment method.
There are many variables that affect.
Investigating the rate of reaction between marble chips and the varying concentrations of hydrochloric acid planning before starting the experiment i had to work out how much marble chips would be needed and how much hcl would be needed.
This experiment is to show how much carbon dioxide is produced during the reaction between an acid hydrochloric acid and marble.
The rate of this reaction can be changed by changing the size of the marble chips.
An outline of an experiment that could be used to find the time and hence rate of reaction of marble chips and hydrochloric acid.
Hydrochloric acid 20ml 0 5m 1m 2m marble chips 2g per test large measuring cylinder plastic bowl 3 4 full of water rubber tubing glass conical flask stopwatch method.
Measured out 1ml of water in a 10ml measuring cylinder and placed into the test tube labelled 2.
Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
Measured 5ml of hydrochloric acid in the 10ml measuring cylinders and placed into each beaker separately.
Marble chips placed onto pieces of paper.